ABSTRACT
The national spontaneous adverse event (AE) monitoring system administered by the Health Sciences Authority (HSA) is supported by a network of healthcare professionals, who actively report adverse reactions of health products observed in clinical practice.

When a new health product is submitted for registration, the demonstration of its efficacy and the evaluation of its safety are generally based on a limited number of patients in clinical trials. In addition, the exclusion of certain patient groups in clinical trials, the lack of significant long-term treatment experience, and limitation of concomitant therapies do not allow a thorough evaluation of the safety profile. Under such circumstances, the detection or confirmation of rare AEs is particularly difficult during the pre-registration development of the product. In order to develop a comprehensive picture of clinical safety, marketed health products have to be closely monitored for their safety when used in actual practice.
Surveillance of health products is an important aspect of the continual benefit-risk assessment of the product. Surveillance activities include mandatory reporting from pharmaceutical manufacturers, spontaneous reporting from health professionals, literature reviews and the exchange of regulatory information with other national drug regulatory bodies.
Among these approaches, the spontaneous AE reporting by the health professionals forms the cornerstone of post-marketing safety surveillance. It remains one of the most important ways of monitoring the safety of a health product throughout its marketed life.
When an AE occurs, the assessment of the possible contributory roles of a health product is an important part of the clinical diagnostic process. This is valuable information that many physicians have shared with HSA over the past years, which has enabled us to identify key safety signals from the local use of marketed health products.
INTRODUCTION
An adverse event (AE) is defined as any untoward medical occurrence that may present during treatment with a health product but which does not necessarily have a causal relationship with this treatment. It excludes AE caused by accidental or deliberate overdoses and medication errors.
HSA encourages all healthcare professionals to report AEs experienced by your patients especially those that are suspected to be associated with a health product. Reporting an adverse event does not necessarily mean that there is a definite link between the event and the health product.
WHAT TO REPORT?
Report any serious and unexpected adverse events to marketed health products.
Health products include:
- Prescription drugs and over-the-counter (OTC) medicines.
- Vaccines and other biologics.
- Medical devices.
- Complementary medicines such as traditional Chinese medicines, Malay traditional medicine, (e.g. Jamu) and Health Supplements.
- Cosmetics.
- In particular, please report
-
All serious and non-serious adverse events to recently marketed health products that are new in the
Singapore - All serious adverse events to established drugs, even if the reactions are well known. This allows us to give advice on how the drug can be used more safely in clinical practice.
- All unexpected events to established drugs, i.e. adverse reactions that are not listed in the product package insert or labelling.
- All serious adverse events to complementary and herbal remedies.
WHAT IS A SERIOUS ADVERSE EVENT OR REACTION?
A serious adverse event or reaction is one which leads to the following outcomes:
- Death or Life-threatening
Report if you suspect that the death was an outcome of the AE or the patient was at a high of risk of dying at the time of the AE, or the continued use of the product (e.g. device) would have resulted in the death of the patient.
- Hospitalisation (Initial or prolonged)
Report if you suspect that the patient was warded at the hospital or had prolonged hospitalisation as a result of the AE.
- Disability or Permanent Damage
Report if the AE resulted in a significant, persistent or permanent impairment or disruption in the patient’s body function or quality of life.
- Birth Defect
Report if you suspect that exposure to a medical product prior to conception or during pregnancy may have resulted in the adverse outcome in the child.
- Other Medically Significant/Important Events
Report when the AE might not be immediately life- threatening or result in death or hospitalisation but might jeopardise the patient or require intervention to prevent one of the other outcomes listed in the definition above. Examples of such events are emergency treatment for allergic bronchospasm, blood dyscrasias or convulsions that do not result in hospitalization, or development of drug dependency or drug abuse.
HOW MANY AE REPORTS HAVE HSA RECEIVED?
In 2010, HSA’s Vigilance Branch reviewed a total of about 11,200 reports and 45% of these were classified as serious reactions. Majority of reports analysed were associated with pharmaceuticals/biologics (97.6%) followed by vaccines (1.9%) and complementary medicines (0.5%).
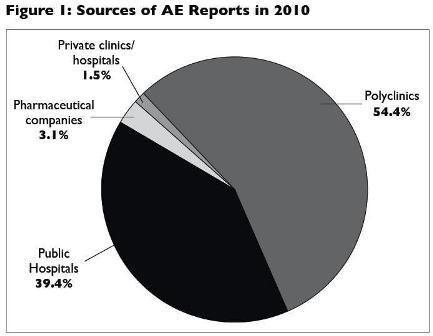
Most of the reports were from healthcare professionals working in the government clinics (54.4%), followed by public hospitals (39.4%), pharmaceutical companies (3.1%) and private clinics/hospitals (1.5%). See Figure 1.
WHAT TYPE OF INFORMATION IS COLLECTED ON AE REPORTING FORMS?
When reporting an AE, please fill in as much information as you know in order to help us assess causality of the adverse reaction. Do not refrain from reporting because some details are not known. Be assured that patient’s and reporter’s identities are kept in strict confidence.
The following information is required -
- Patient’s particulars (e.g. initials, age, gender).
- Reporter’s details (e.g. Name, place of practice, contact number).
- Details of adverse event (e.g. description, date of first occurrence of event).
- Suspect health product(s) (e.g. brand name or active ingredient(s), dose, period of intake).
- Concomitant health product(s) (e.g. including complementary medicines).
- Outcome of the patient (e.g. seriousness of event; Was patient hospitalised; Has patient recovered?).
- Treatment given to patient.
- Other relevant information (e.g. known allergies, lab test report).
SUBMIT YOUR AE REPORTS ONLINE - A FASTER AND EASIER OPTION
For faster and easier reporting, you are encouraged to submit AE reports online at the following HSA’s website: www.hsa.gov.sg/ae_online
Alternatively, you may download the AE reporting forms for health products at the same website and submit them to the Vigilance Branch of HSA:
- Email address: HSA_productsafety@hsa.gov.sg
- Fax: 6478 9069
- Phone: 6 866 3538/9
Any follow-up information for an AE that has already been reported can be sent to us on another form or can be reported via the other available modes of reporting. Please indicate that it is a follow-up report. It is very important that follow-up reports are identified and linked to the original report.
Upon submission of an AE report, the reporting doctor can request information on the suspected health product or ask to be updated on the investigation or causality assessment conducted by the Vigilance Branch of HSA by providing us with his contact details.HOW TO GAIN ACCESS TO HSA AE DATABASE?
All registered pharmacists and doctors can gain access to anonymised case reports online at HSA’s AE enquiry database via your MCR no or SingPass at the following website:
MOH’s Health Professional Portal (HPP): http://www.hpp. moh.gov.sg/HPP/HPP_Home.html
HOW WOULD YOUR REPORT CONTRIBUTE TO SAFETY SURVEILLANCE OF HEALTH PRODUCT?
Each report is individually reviewed with more attention spent on the serious AE reports. The information is then entered into the computer database for aggregate analysis.
The AE reports may identity unexpected adverse events or indicate that certain adverse events occur more commonly than previously expected, or that some patients are more susceptible to some problems than others. Such findings can lead to changes in the marketing authorisation, for example restrictions in use, refinement of those instructions or the introduction of specific warnings of adverse events in product package insert. In rare circumstances, when a hazard is considered unacceptable, a registered health product may have to be withdrawn from the market.
HSA also contributes to the international surveillance of drug safety signals as a member of the World Health Organisation (WHO) International Drug Monitoring Programme, by submitting these anonymised AE reports to the WHO-Uppsala Monitoring Centre in
WHAT ARE THE TYPES OF SAFETY INFORMATION AND HOW ARE THEY COMMUNICATED?
Examples of safety information:
- Withdrawal or suspension of a health product.
- Major safety issues associated with the use of a health product.
- Safety information which may have significant impact on clinical practice.
- Interim safety updates and regulatory positions while evaluating emerging safety signals associated with a health product.
- Adulteration of a health product.
- Dear Healthcare Professional Letters at HSA/ MOH- Health professionals Portal (MOH-HPP) Website: To login to the secured webpage http://www.hpp.moh.gov.sg/
- Adverse Drug Reaction (ADR) News Bulletin: www.hsa.gov.sg/adrbulletin
- HSA Product Safety Alerts and Interim Safety Updates http://www.hsa.gov.sg/pdt_safetyalerts
- Press Releases: http://www.hsa.gov.sg/press_releases
- Package Insert Updates: http://www.hsa.gov.sg/label_amend
LEARNING POINTS
- Reporting AE contributes to continued surveillance of health product’s safety because there is
- limited safety information surrounding a health product when it is introduced into the market.
- HSA encourages all healthcare professionals to report all AEs , even if the role of the suspected product in the AE is yet to be ascertained.
- Patient’s and reporter’s identities are kept in strict confidence.
- Report AEs on-line via HSA’s website for convenience.
- All registered pharmacists and doctors can gain access to anonymised case reports online at HSA’s AE enquiry database via your MCR no. or SingPass through the MOH-HPP Portal.
- Contributed by:
BELINDA TAN, DOROTHY TOH, SALLY SOH
Vigilance Branch, Health Products Regulation Group
Health Sciences Authority
**********


