Here are some brief pharma news for your speedy update:
• Indian Companies Top US Generic Approvals
• US FDA’s Deputy Commissioner Resigned
• Indian Companies Top US Generic Approvals
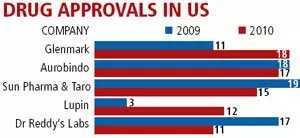
The biggest players in US generic drugs market are not home bred companies, but Indian. With a lion’s share of 33.2% Glenmark, Aurobindo & Sun Pharma bagged the biggest approvals of generic drugs approved by US FDA in 2010. These companies are likely to replace the former top generic makers, also fellow Indian comapanies like Ranbaxy & Dr Reddy’s.
Glenmark received the highest generic approvals in 2010, trailed closely by Aurobindo & Sun Pharma respectively. Lupin is an emerging player in US market as it registered the highest increase in the generic approvals in 2010. See chart below.
• US FDA’s Deputy Commissioner Resigned
Less then 2 years into his powerful job, Josh Sharfstein is leaving FDA to take on the post of Secretary of Health & Mental Hygiene in Maryland. Just as FDA was facing a number of safety & regulatory issues, his departure comes at a time that symbolizes FDA’s change in attitude towards the direction the world’s largest drug regulatory agency was grappling with in order the balance the public health vs. the space for science & business to operate. One of the high profile regulatory decisions made by FDA was to allow the controversial Avandia OHGA drug to remain on the market with prescribing restrictions. During Sharfstein term, the FDA was also more ready to issue warning letters & expressed the agency’s willingness to pursue executives from big pharma for infractions.
**********


